Documents
Datasheets
Download:Description
Pall Kleenpak liquid capsule filters with intrinsically hydrophilic positive zeta potential Posidyne membrane are rugged, self-contained sanitary filters designed for small-batch sterile filtration of aqueous pharmaceutical solutions. With a net positive charge in most aqueous solutions from pH 3 to 10, Kleenpak Capsules with Posidyne Membrane Assemblies can effectively remove contaminants such as cell debris and endotoxin smaller than the filter rating.
Features and Benefits
- Positive Zeta potential for enhanced efficiency in aqueous solutions
- Double-layer sterilizing membranes for high reliability
- 100% integrity and pressure-tested
- High protein recovery from serum and most protein solutions
- 0.2 µm NF grade for sterilization
- 0.1 µm NT grade for diminutive bacteria and mycoplasma removal
- Low filter extractables
- Sanitary vent and drain valves with non-twist hose barbs
- Upstream hold-up volumes (< 1 mL)
- Repeatedly autoclavable
- Manufactured for use in conformance with cGMP
- IS0 9000 Certified Quality System
- Pharmaceutical P optimized
- Certificate of Test provided
Quality and Bio-Safety Biological Tests
- Meets USP Biological Reactivity Test, in vivo, for Class VI-121 °C Plastics
Effluent Quality Tests*
- Meets Cleanliness per USP Particulates in Injectables
- Non-Fiber-Releasing
- Non-Pyrogenic per USP Bacterial
- Endotoxins (< 0.25 EU/mL)
- Meets Total Organic Carbon and Water Conductivity per USP Purified Water; pH per Sterile Purified Water
* Per lot sample soak or rinse-up flush aliquots.
Autoclave Resistance
- Lot samples multi-cycle autoclave challenged
Specifications
Materials of Construction
| Membrane | Nylon 6,6, positively-charged |
| Support and Drainage | Polyester |
| Core and Cage | Autoclavable Version: Polypropylene G and S Grades: Polyester |
| End Caps and Shell | Polyester |
| Vent and Drain Valve O-ring | Ethylene Propylene (EPDM) |
Microbial Removal Ratings
| NFZ | 0.2 µm, sterilizing-grade1 |
| NTZ | 0.1 µm, sterilizing-grade2 |
2 Also retains > 107 cfu/cm2 Acholeplasma laidlawii mycoplasma.
Nominal Filter Areas
| Size Code | KA1 | KA2 | KA3 | KA4 |
| Effective Filter Area | 0.05 m2 (0.53 ft2) |
0.1 m2 (1 ft2) |
0.2 m2 (2.1 ft2) |
0.5 m2 (5.3 ft2) |
Nominal Dimensions
| Size Code | KA1 | KA2 | KA3 | KA4 |
| Maximum Diameter of Bowl (including Valves) |
94 mm |
94 mm (3.7 in.) |
109 mm (4.2 in.) |
109 mm (4.2 in.) |
| Length (including Sanitary Connection [Code 1]) | 117 mm (4.6 in.) |
158 mm (6.2 in.) |
174 mm (6.8 in.) |
286 mm (11.2 in.) |
| Length (including Stepped Hose Barb Connection [Code 2]) | 158 mm (6.2 in.) |
199 mm (7.8 in.) |
- | - |
| Length (including Hose Barb Connection [Code 6]) | - | - | 210 mm (8.2 in.) |
325 mm (12.7 in.) |
Operating Conditions3
| Maximum Pressure and Temperature | 5.2 barg (75 psig) to 40 °C |
| Maximum Differential Pressure | 4.1 bard (60 psid) at 40 °C |
Typical Liquid Flow Rates4
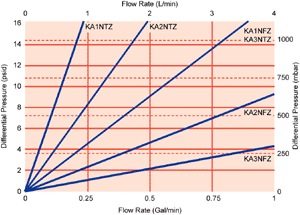
4 Typical initial clean ∆P, water at 20 °C, 1 cP. KA4NFZP ∆P flow is 37 mbard/L per min (2.1 psid/gpm). Pressure drops at other flows are directly proportional to flow. Values shown for 38 mm (1½ in.) sanitary flange connections. Values with other connections are available on request. For assistance, contact your local Pall representative.
Aqueous Extractables (NVR)5 (Non G and S Grades)
| KA1 | KA2 | KA3 | KA4 |
| 6 mg | 12 mg | 20 mg | 40 mg |
Steam Autoclaving6 (Non G and S Grades)
| Cumulative Autoclave Time | 16 hours at 125 °C 4 hours at 140 °C |
Warning: Kleenpak filters must not be steam-sterilized in situ by passing steam through under pressure.
Sterilization
| Gamma-irradiation Maximum Dosage (G Grade) | 35 kGy |
Type
Filter Capsules
Use
Sterilizing-Grade Liquid Filtration
Related Documents
Application Note: Successful Wetting for Filter Integrity Testing in Volume-Restricted Systems
Regulatory/Quality Documentation
Product Summary
Kleenpak™ Capsules with Posidyne® Membrane meet cleanliness requirement per USP Particulates in Injectable. It features positive Zeta potential for enhanced efficiency in aqueous solutions. Capsules contain a double-layer sterilizing membrane for high reliability and high-quality product. Filters are self-contained and designed for small sterile filtration systems throughout a range of acidic to basic solutions.
Reviews
We appreciate your review of this product. Please login to your account to leave a review.