21 Results of 21 total products











Datasheets
Download:Kleenpak capsules are rugged and compact, can be supplied with a broad range of filter media, and can be offered in up to four different filter area sizes designed for a variety of applications in pharmaceutical manufacturing environments (volumes of 30 L to 1000 L) from small to large-scale. These in-line single-use assemblies are available with sanitary or hose barb inlet/outlet fittings.
Key Features and Benefits
- Encapsulated format for higher flexibility, minimized cleaning and low installation costs
- Built in prefiltration
- Minimal hold up volume
- Low extractables
- High protein transmission
- Rapid preservative recoveries
- Easy integrity testing
- Compatible with organic solvents, acids and chemicals¹
- Resin and surfactant-free
- Melt-sealed, non shedding
¹ Except ketones and amides
Quality Standards
- Manufactured for use in conformance with cGMP
- 100 % integrity tested
- ISO 9000 Certified Quality System
- Meets USP Biological Reactivity Test in vivo for Class VI-121 °C plastics
- Every filter tested during manufacture
- Test correlated to microbial retention
- Certificate of Test provided includes
- Fabrication Integrity
- Bacterial Retention
- Material of Construction
- Effluent quality for cleanliness, TOC and water conductivity, pH and pyrogens
Materials Of Construction
| Filter Membrane | Hydrophilic modified PVDF |
|
Support/Drainage |
Polypropylene Polypropylene Polypropylene |
| Sealing Technology | Thermal bonding without adhesives |
Operating Parameters¹
| Maximum Operating Temperature | 40 °C |
| Maximum Operating Pressure | 5.2 bar (75 psi) at 20 °C 4.0 bar (58 psi) at 40 °C |
| Maximum Differential Pressure (Forward Direction) | 4.0 bar (58 psi) at 40 °C |
Sterilization²
| Autoclave (G and blank option only) | 30 x 60 minutes at 125 °C 10 x 60 minutes at 140 °C |
| Gamma Irradiation (G option only) | Maximum of 50 kGy |
Typical Extractables in Water at 20 °C³
| < 5 mg per capsule |
Nominal Dimensions
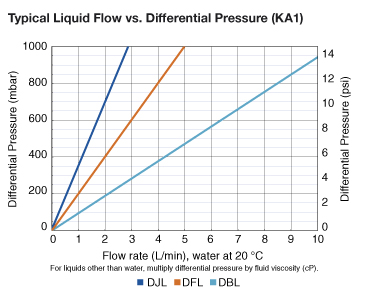
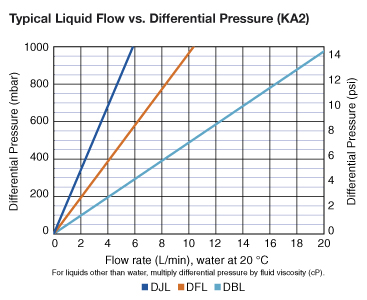
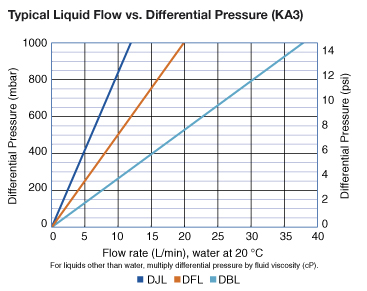
| KA1 | KA2 | KA3 | KA4 | |
| Diameter incl. Valves | 94 mm (3.7 in.) | 94 mm (3.7 in.) | 105 mm (4.1 in.) | 105 mm (4.1 in.) |
| Length - Code 1 | 117 mm (4.6 in.) | 157 mm (6.2 in.) | 174 mm (6.8 in.) | 287 mm (11.3 in.) |
| Length - Code 2 | 158 mm (6.2 in.) | 198 mm (7.8 in.) | - | - |
| Length - Code 6 | 157 mm (6.2 in.) | 197 mm (7.7 in.) | 210 mm (8.3 in.) | 327 mm (12.8 in.) |
| Length - Code 12 | 137 mm (5.4 in.) | 177 mm (7.0 in.) | - | - |
| Length - Code 16 | 137 mm (5.4 in.) | 177 mm (7.0 in.) | 192 mm (7.6 in.) | 305 mm (12.0 in.) |
Nominal Effective Filter Area (EFA)
| KA1 | 400 cm² (0.4 ft²) |
| KA2 | 800 cm² (0.9 ft²) |
| KA3 | 1500 cm² (1.6 ft²) |
| KA4 | 3300 cm² (3.6 ft²) |
Regulatory/Quality Documentation

We appreciate your review of this product. Please login to your account to leave a review.