1/EA
1
Datasheets
Download:Supor® Beverage Filter Cartridges
For Final Filtration
Supor Beverage filter cartridges are hydrophilic membrane filters designed for reliable retention of spoilage microorganisms in the final filtration of a range of beverages including wine and water.
Description
The Supor Beverage filters were developed and validated for removal of common spoilage microorganisms.
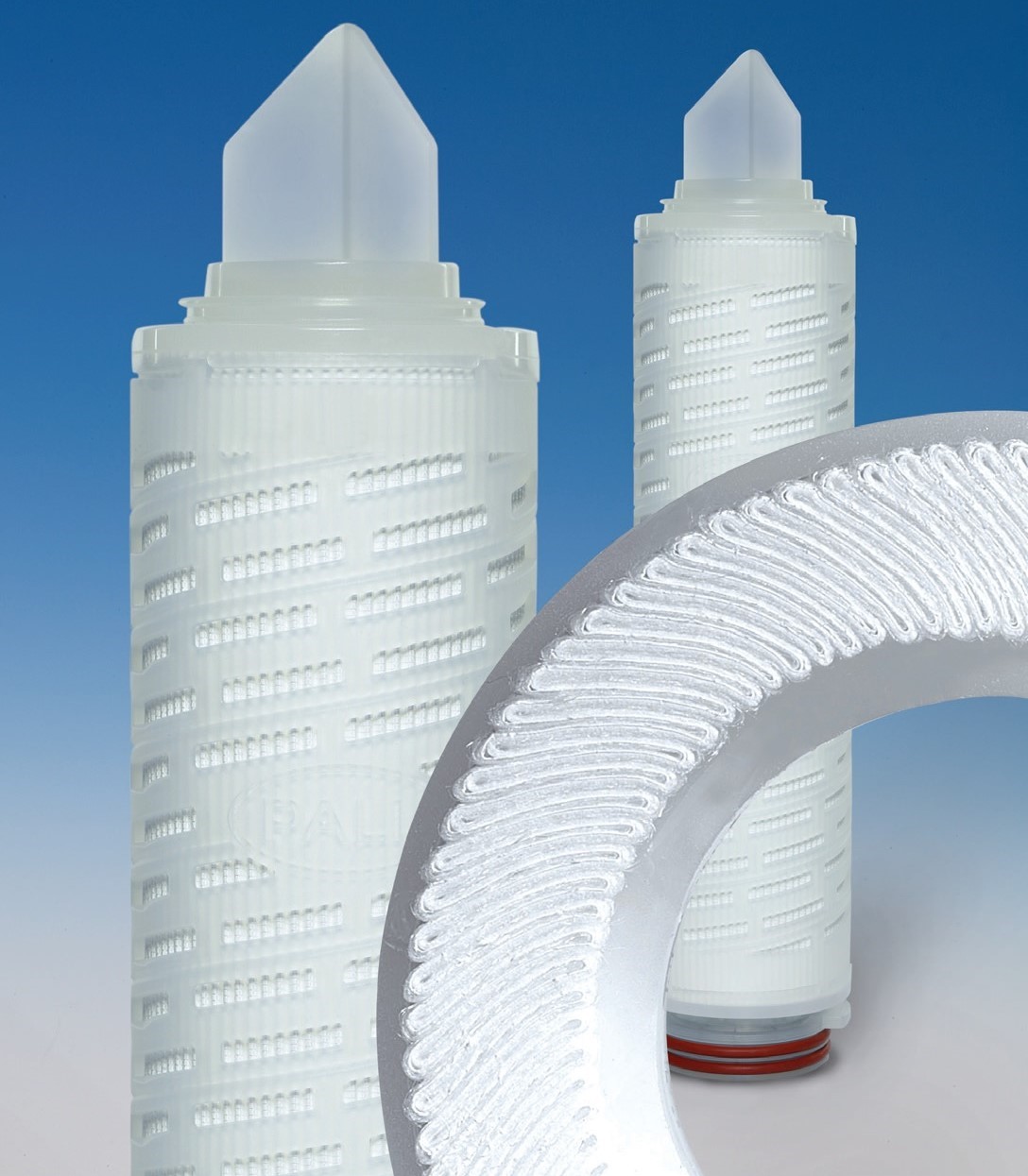
The cartridges are constructed from one layer of polyethersulfone (PES) membrane in a laid-over pleat configuration. The single open ended (SOE) configuration is designed to fit into sanitary housings to ensure effective microbial reduction and assembly integrity.
Supor Beverage filter cartridges are suitable for exposure to repeated hot water sanitization and in situ steam sterilization cycles for longer service life. The laid-over pleat configuration combined with optimized support and drainage materials, provide increased mechanical strength during operation, repeated hot water, chemical and steam sanitization and thus, high throughput.
| Features | Benefits |
| Inert polyethersulfone (PES) media |
|
| Cartridges resistant to numerous sanitization cycles |
|
| Hydrophilic membrane |
|
| Validated with wine spoilage microorganisms |
|
| Individually serialized cartridges |
|
| Integrity testable |
|
| Multiple adaptor options |
|
Quality
- Cartridges produced in a controlled environment
- Manufactured according to ISO 9001:2015 certified Quality Management System
Food Contact Compliance
Please refer to the Pall website http://www.pall.com/foodandbev for a Declaration of Compliance to specific National Legislation and/or Regional Regulatory requirements for food contact use.Microbial Removal Rating
| Test Organism | Log Reduction Value (LRV) for BB Grade | Log Reduction Value (LRV) for BK Grade |
| Serratia marcescens (ATCC 14756) | >10 | |
| Oenococcus oeni (ATCC 23279) | >8^ | |
| Escherichia coli (ATCC 25922) | >10 | |
| Saccharomyces cerevisiae | Yeast free* | |
| Dekkera bruxellensis (ATCC 64276) | Yeast free* | |
Challenges were performed at a level of ≥107 per cm2 of effective filtration area on new and unused filters.
^ For O. oeni challenges were performed at a level of ≥105 per cm2 of effective filtration
area on new and unused filters.
* Filters provided a yeast-free effluent when challenged.
Materials of Construction
| Filter medium* | Polyethersulfone (hydrophilic) |
| Support and drainage | Polypropylene |
| Core, Cage, End Cap and Fin End | Polypropylene |
| Adaptor | Polypropylene with stainless steel reinforcing ring |
| O-ring Seal | Ethylene propylene rubber or Silicone elastomer |
* Each 10” module contains 0.77 m² (8.3 ft²) of effective filtration area for BB grade and 0.75 m² (8.0 ft²) of effective filtration area for BK grade.
Technical Information
Operating Characteristics in Compatible Fluids1
| Maximum Differential Pressure | Operating Temperature | |
| 5.5 bard (79.8 psid) (forward pressure) | 25 °C (77 °F) | |
| 4.0 bard (58.0 psid) (forward pressure) | 80 °C (176 °F) | |
| 1.0 bard (14.5 psid) (reverse pressure) | 40 °C (104 °F) | |
| 300 mbard (4.4 psid) (forward pressure) | during in-situ steam sterilization | |
1Compatible fluids are defined as those which do not swell, soften or attach any of the filter components
Sterilization and Sanitization
| Media | Temperature | Cumulative Exposure Time/cycles2 | |
| BB Grade | BK Grade | ||
| Steam | 125 °C (257 °F) |
125 x 20 min cycles |
125 x 20 min cycles |
| Hot Water | 90 °C (194 °F) |
100 hours 200 x 30 min cycles |
200 x 30 min cycles |
| Peracetic acid (PAA), 325 ppm PAA (1275 ppm H2O2to give 1600 ppm of total peroxides) |
ambient | 1000 hours | 2000 hours |
| Potassium metabisulphite (1000 ppm) |
ambient | 1000 hours | 1000 hours |
2 Measured under laboratory test conditions. The actual cumulative time depends on the process conditions. For applications requiring sterilization or sanitization Pall recommends the use of Code 7 adaptors to ensure filter sealing after cooling. Cartridges should be cooled to system operating temperature prior to use. Contact Pall for recommended procedures.
Chemical Cleaning (static soak conditions)
| Media | Temperature | Cumulative Exposure3 | |
| BB Grade | BK Grade | ||
| Caustic 2% | 50 °C (122 °F) | 200 hours | 400 hours |
| Caustic 2% | 80 °C (176 °F) | 100 hours | 200 hours |
3 Measured under laboratory test conditions. The actual cumulative time depends on the process conditions.
Pressure Drop vs. Liquid Flow Rate4
| Code | Value |
| BB | 30 liters per minute @ 100 mbar (5.4 US gpm @ 1 psi) |
| BK | 42.5 liters per minute @ 100 mbar (7.6 US gpm @ 1 psi) |
4 Typical initial clean media differential pressure (dP) per 254 mm (10”) cartridge for water at 20 °C (68 °F); viscosity 1 centipoise. For 508, 762 mm and 1016 mm configurations, divide the differential pressure by 2, 3, and 4, respectively.
Ordering Information
Cartridge Part Number
AB [Table 1] S [Table 2] [Table 3] W [Table 4]
This is a guide to the Part Numbering structure only. For specific options, please contact Pall.
Table 1: Nominal Length
| Code | Description |
| 1 | 254 mm (10”) |
| 2 | 508 mm (20”) |
| 3 | 762 mm (30”) |
| 4 | 1016 mm (40”) |
Table 2: Removal Rating
| Code | Description |
| BB | 0.45 μm |
| BK | 0.65 μm |
Table 3: Adaptor
| Code | Description |
| 3 | SOE – single open end with flat closed end and external 222 O-rings |
| 7 | SOE – single open end with fin end, 2 locking tabs and external 226 O-rings |
| 8 | SOE – single open end with fin end and external 222 O-rings |
| 28 | SOE – single open end with fin end, 3 locking tabs and external 222 O-rings |
Table 4: O-Ring Seal Material
| Code | Description |
| H4 | Silicone Elastomer |
| J | Ethylene Propylene Rubber |
We appreciate your review of this product. Please login to your account to leave a review.